-
- Market Research
- |
- CBD Near Me
- |
- Giveaways
- |
- Newsletter
- |
- Contact
- |
- Advertise
- |

In the pre-FDA purgatory that is the hemp industry, the US Hemp Authority certification provides the highest level of legitimacy possible to hemp suppliers and brands that can meet the organization’s high standards.
When reviewing CBD brands, we reward heavy credit under the “certifications” factor when we see the US Hemp Authority seal because it represents a brand’s commitment to safety, transparency, and FDA-ready standards like cGMP (current good manufacturing practices) certification.
But how does this hemp industry certifying body hold brands accountable to these standards?
To learn more about the background of the program, their certification process, and the new standards update (Program Standard 3.0), we spoke with Dr. Marielle Weintraub, the President of the organization.
A Conversation With the US Hemp Authority President
Preserving the potential of hemp products and the integrity of the parties who sell them has been a key priority since day one, Dr. Weintraub explained to us.
Without FDA regulation, the hemp industry is vulnerable to its own bad actors, which is what drove the more policy-focused US Hemp Roundtable to invest in a self-regulating body.
Thus, in 2017, the US Hemp Roundtable seeded the US Hemp Authority because “they were concerned at the time that somebody in the industry would have what we call a ‘60 minutes moment’ and bring it all down before we could show consumers and regulators what hemp can really do,” Dr. Weintraub told us.
The US Hemp Authority is now completely independent of the US Hemp Roundtable, and is committed to giving consumers access to high-quality products from transparent brands that are well-prepared for eventual FDA regulation.
To meet this rather tall order, the group has written and rewritten their standards multiple times since their inception in 2017.
Program standards 1.0 came out in 2018, and the original crop of 13 certified brands (who were audited using a third party organization) officially received their seals in March of 2019.
Thanks to a bit of pushback from opponents of the original standards and the quickly shifting hemp industry itself, the US Hemp Authority soon realized their standards would need to be continually refined and updated.
To meet this challenge, they formed a technical committee made up of industry stakeholders, trade associations, and more, which informed many of the changes for version 2.0 of their standards.
“The idea was to continue to learn from the initial launch, receive greater input, improve the program, and evolve alongside the hemp industry,” said Dr. Weintraub.
The latest iteration of this ongoing effort is version 3.0 of the standards, which is largely focused on clarifying and refining the guidance set forth in version 2.0.
The New Rule: Program Standards 3.0
Slashing the document from 80 pages to 23 pages in the 2.0-3.0 transition may seem drastic at first, but Dr. Weintraub assured us the aim was to clarify, not omit or restructure the fundamentals (as was more the case from 1.0 to 2.0).
She listed the most prominent changes to version 3.0 as follows:
- Introduction added
- Glossary terms redefined
- Product testing and cGMP requirements clarified
- Labeling rules updated

Many of the fundamental changes informed by public input and the technical expertise of the US Hemp Authority’s committee were established in the 1.0 to 2.0 transition, which is why this go around was more about clarification.
Overall, the standards oversee several dozen factors designed to comprehensively assess a product’s safety and transparency, which entails setting requirements for every step of the supply chain.
This means holding growers, processors and manufacturers, and brand owners accountable to high standards of safety and transparency that will readily comply with eventual FDA regulations.
“When it comes to manufacturers, we want to make sure they’re cGMP certified if they’re making dietary supplements or food, as that will be a requirement when FDA is officially overseeing us as an industry,” explained Dr. Weintraub.
The clarifying of definitions, especially surrounding “broad spectrum” and “full spectrum,” was largely intended to hold brands more accountable for their claims.
For example, under the 3.0 definition of broad spectrum, a brand can’t simply use poorly calibrated laboratory equipment and then claim their product is THC free; the “limit of quantification” has to be less than 0.01%.
When we asked how the program standards pivot to address controversial trends in the industry like the rise of delta-8 THC and proprietary hemp extraction methods, Dr. Weintraub brought the focus to end product quality.
“We’re not here to squash innovation; we have no reason to do so. It’s what makes our industry great,” she said. “However, we are checking the finished product to make sure it is compliant on the quality side.”
This is what allows the US Hemp Authority the flexibility to welcome new innovations without budging on their standards; it’s up to brands to prove that their innovative products and processes are good enough to pass.
However, delta-8 THC still strikes out on two fronts.
First, the US Hemp Authority does not certify products that focus their marketing around the intoxicating effects of cannabinoids like THC (state-required reporting of THC level is allowed).
Even if the delta-8-containing product is not being marketed in this way, synthetic and biosynthetic cannabinoids are explicitly prohibited, a decision that protects hemp farmers (who would be ousted by orange peels) and consumers alike while preparing for FDA standards.
Looking to the Future
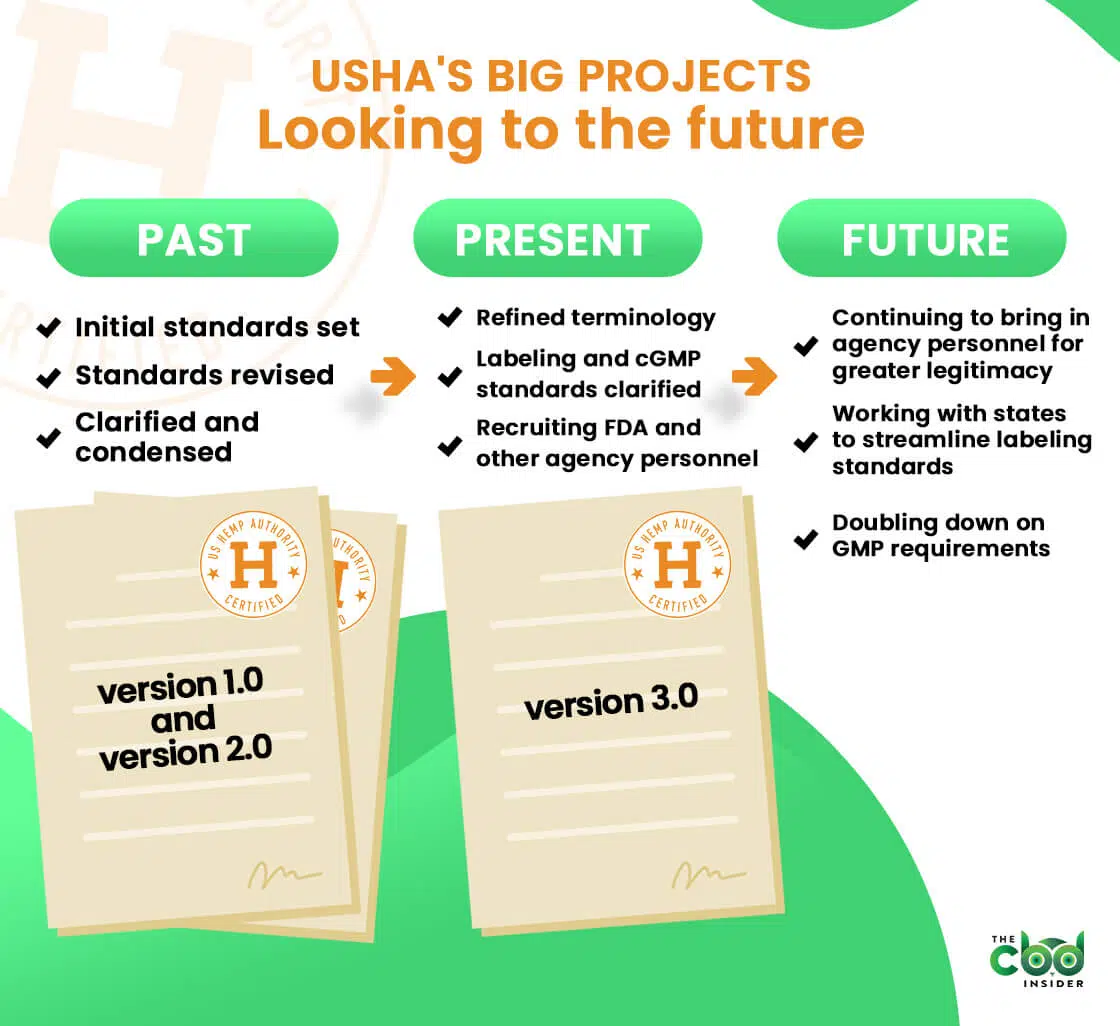
All of these much-needed clarifications aside, 3.0 is not likely to be the last we hear of the US Hemp Authority.
The fact that they’re still adding new board members—several of which are from federal agencies and trade organizations—speaks of a commitment to further progress the state of hemp certification while addressing public concern surrounding hemp products.
One such board member—Larisa Pavlick, VP of Global Regulatory & Compliance at the United Natural Products Alliance (UNPA)—was kind enough to share her thoughts on how the hemp industry (manufacturers, suppliers, and brands) can prepare themselves for FDA regulation.
“There really is no excuse for not following GMPs (good manufacturing practices),” she told us. “The first step is to register with the FDA if producing a food or ingestible product… before distributing products into the consumer market.”
In addition, Pavlick was passionate about training and education for the Food Safety Modernization Act (as well as GMPs), noting that the UNPA offers a course “designed to help the dietary supplement company implement the regs and prepare for a regulatory inspection.”
The constantly shifting landscape of state-level hemp legislation is another evolving issue that may significantly impact the future of the hemp industry.
To prevent this problem from spreading into a regulatory landscape that makes interstate/online commerce a huge pain in the CBD industry, USHA is currently in an ongoing effort to reconcile labeling and other requirements across states so that they don’t obfuscate or conflict with USHA certification requirements.
For more insight on what the future of hemp may hold, we asked Dr. Weintraub that exact question.
“What makes the hemp industry great is that people can get in pretty easily, but it’s going to be like the dietary supplement industry. There are only so many gel cap and gummy manufacturers. There’s quite a lot of white labeling going on, and that will probably be reduced as we get more and more regulated and/or the FDA takes over. We will see the companies who followed cGMP and invested more into testing and safety standards survive.”
As close watchers of the industry striving for the same end, it reassured us to hear that the leading certifier of hemp products continues to prioritize consumer safety and education with every change they make.
Visit the US Hemp Authority.







